Mumbai: Wockhardt Ltd. has achieved a major milestone as the US Food and Drug Administration (FDA) formally accepted its New Drug Application (NDA) for Zaynich, a first-in-class antibiotic. Filed on September 30, this marks the first time an Indian company’s New Chemical Entity has been accepted by the US regulator, signaling a historic moment for the Indian pharmaceutical sector.
Zaynich: Tackling Resistant Infections
Zaynich is a novel antibiotic designed to fight resistant bacterial infections, an area where global drug innovation has been limited. Wockhardt said that the FDA’s acceptance reflects the scientific strength and innovation behind the drug, underscoring India’s growing capability in new drug development beyond its traditional strength in generics.
A Big Step for India’s Pharma Industry
The company emphasised that this NDA acceptance is not only a milestone for Wockhardt but also a major breakthrough for the Indian pharmaceutical industry. For years, Indian pharma has been globally recognised for generics, but new drug discovery has been a challenging area. Zaynich represents a step forward in bringing Indian innovation to the global stage.

Shares React Positively
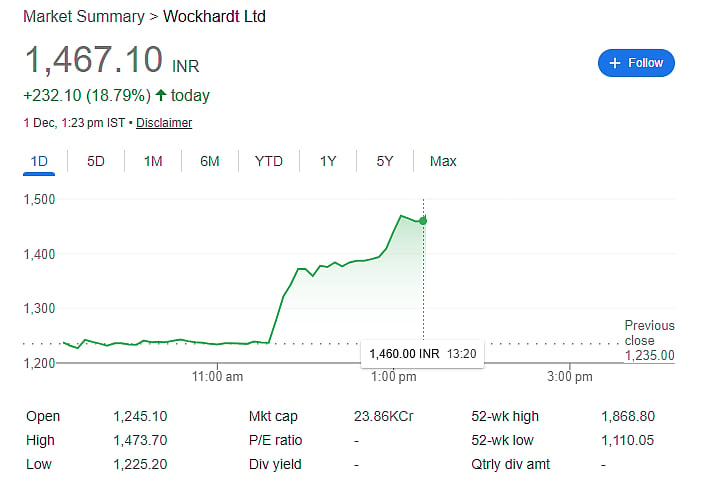
Following the announcement, Wockhardt shares jumped over 19 percent, trading at Rs 1,386.90. Despite the gain today, the stock has still recorded a 5 percent decline in 2025. Investors are optimistic about the potential commercial success of Zaynich and the company’s regulatory progress.
Next Steps
Wockhardt remains committed to advancing Zaynich through the FDA’s regulatory process. The company aims to deliver a new antibacterial solution to global markets, especially at a time when antimicrobial resistance is a growing concern worldwide.