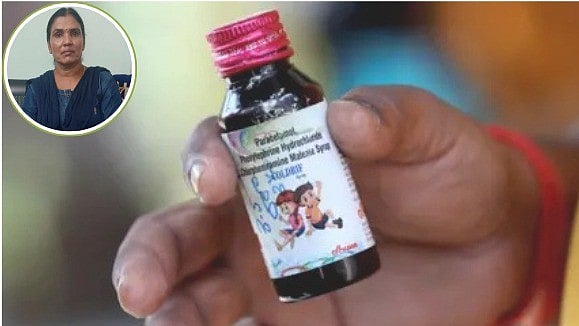
Bhopal (Madhya Pradesh): Pharmaceutical manufacturing companies have been instructed to ensure batch-wise testing of raw materials and finished products, following reports of child deaths linked to contaminated cough syrup.
MP drug controller Dinesh Kumar Srivastav gave the instructions pertaining to quality control in a meeting of the Indian Drug Manufacturers’ Association (IDMA), MP State Drug Manufacturers’ Association (MPSDMA) and Federation of MP Chamber of Commerce and Industry (FMPCCI) on Tuesday.
As per the Food and Drug Administration (FDA), pharmaceutical companies have been instructed toconduct batch-wise testing of raw materials and finished products tocomply with the Indian drug rules.
The companies have also been instructed for entry and on boarding on ONDLS (Online National Drug Licensing System). Warnings and restrictions should be complied with as the government has prohibited cough syrups containing chlorpheniraminemaleate and phenylephrine hydrochloride for children under four years of age due to safety concerns.
The companies have also been instructed to notice changes in provisions ofthese warnings.
The Central Drugs Standard Control Organisation (CDSCO) has alerted state drug controllers to enforce this rule, which requires testing each batch of active and inactive ingredients and the final product before release. This directive was issued after inspections found that some manufacturers were not performing these required tests.