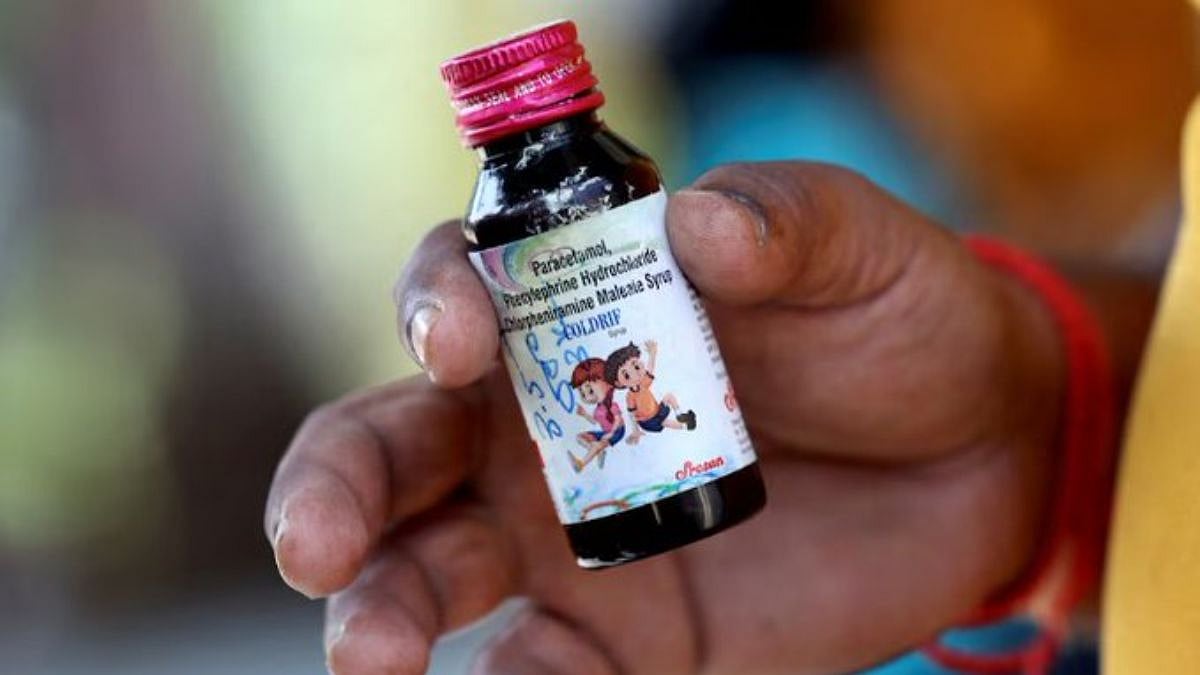
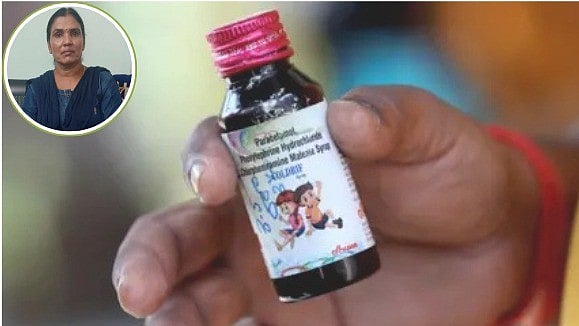
Chhindwara (Madhya Pradesh): After the death of more than 20 children in Chhindwara due to 'toxic' Coldrif cough syrup, irregularities in Ayurvedic medicines have also come to light.
According to information, samples of some ayurvedic medicines were sent to Gwalior for testing by the AYUSH department, where 7 Ayurvedic products failed the quality test.
Following this, products like ‘Giloy Satva’ and ‘Kasamrit Syrup’ have been immediately banned in Chhindwara.
Now, Jabalpur district has also banned the sale of these medicines. The AYUSH department is continuing a detailed inspection across both districts.
Regarding the matter, District AYUSH Officer, Suratna Singh Chauhan, said, "Samples from various companies failed testing and their sale has been stopped."
Singh further added, “7 Ayurvedic products have failed testing. Their sale is banned in the district. Sampling is underway and anyone found selling banned medicines will face action. We are also investigating any sale continuing in Jabalpur and banning the sale.”
The AYUSH officer added that sampling is being done at Ayurvedic stores and medical shops. If anyone is found selling these banned medicines, strict action will be taken.
List of medicines banned for sale
1.Giloy Satva - Sharmaayu Genuine Ayurveda, Datia (Batch No. 005P-1)
2.Kamdhudha Ras - Sharmaayu Genuine Ayurveda, Datia (Batch No. 25117002P-1)
3.Praval Pishti - Shri Dhanwantari Herbal, Himachal Pradesh (Batch No. PPMB-077)
4.Muktashakti Bhasma - Shri Dhanwantari Herbal, Himachal Pradesh (Batch No. MSBB-059)
5.Laxmi Vilas Ras - Dabur India Ltd., Ghaziabad (Batch No. SB00665)
6.Kafkuthar Ras - Dabur India Ltd., Ghaziabad (Batch No. SB00066)
6.Kasamrit Syrup - Shivayu Ayurveda Ltd., Aurangabad (Batch No. KMSL-2501)
The investigation into the Chhindwara incident is still ongoing, and authorities are intensifying checks to prevent further risks to public health.
(Inputs from FP News Service)