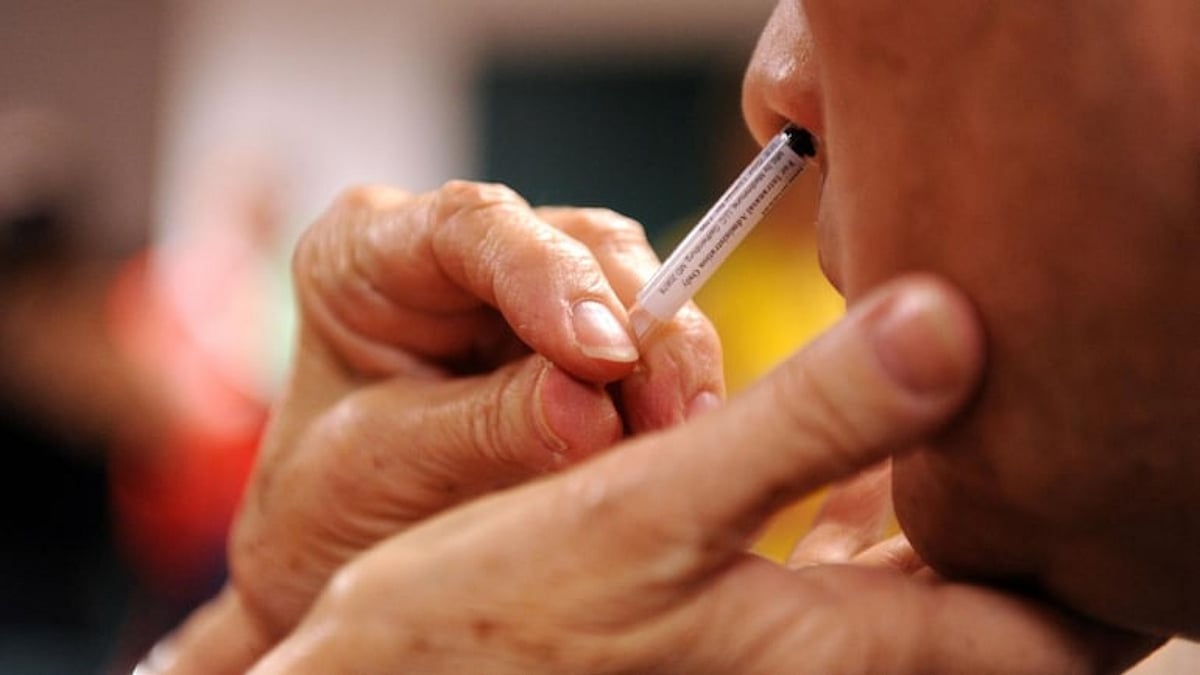
Bharat Biotech's chairman and managing director, Dr Krishna Ella speaking on the status of the nasal vaccine for Covid-19 said, that the bio-pharma company has almost completed phase 2 trials and it has shown good results.
A nasal vaccine could save on medical equipment, such as syringes, and save time taken for vaccination.
"We've almost completed phase 2 trials for the nasal vaccine and it has shown good results. This vaccine will help in controlling the transmission of Covid-19 infection," Dr Krishna Ella was quoted as saying by news agency ANI.
Notably, in January this year, Bharat Biotech had sent a proposal to the Drugs Controller General of India (DCGI) for conducting the phase 1 trials of a nasal vaccine against COVID-19.
COVID-19 vaccine of Bharat Biotech's 'Covaxin' was granted permission for restricted use in an emergency situation by the DCGI on January 3 following which India's mega vaccination drive began on January 16.
Key points of the adenoviral intranasal vaccine BBV154:
1. An intranasal vaccine stimulates a broad immune response – neutralizing IgG, mucosal IgA, and T cell responses.
2. Immune responses at the site of infection (in the nasal mucosa) – essential for blocking both infection and transmission of COVID-19.
3. The nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa.
4. Non-invasive, Needle-free.
5. Ease of administration – does not require trained health care workers.
6. Elimination of needle-associated risks (injuries and infections).
7. High compliance (Ideally suits for children’s and adults).
8. Scalable manufacturing – able to meet global demand.
According to the ministry of science and technology, the Phase 1 trial in healthy volunteers of age groups ranging from 18-60 years was well tolerated. Trials for the Phase 3 trial will commence after the completion of Phase-2 clinical trials.
The intramuscular injection produces antibodies that circulate in the blood to recognize the virus. But this route of administration doesn’t necessarily produce antibodies in the nose and nasal passages.