Payal Banerjee
New Delhi
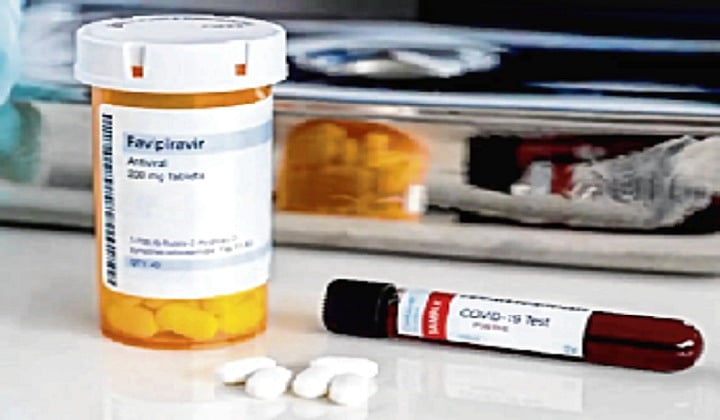
India's drug regulator has approved anti-viral drug favipiravir for "restricted emergency use" in mild to moderate cases of COVID-19, official sources said, as instances of coronavirus infection and fatalities in the country continued its steady upward trend.
Considering the emergency and unmet medical need for COVID-19, the Drug Controller General of India (DCGI) under the fast-tracked approval process granted domestic firm Glenmark Pharmaceuticals the permission to manufacture and market favipiravir (200 mg) tablet.
"The drug has been approved for restricted emergency use in the country for the treatment of mild to moderate cases of COVID-19," an official source in the know of developments told PTI.
Following this approval, Mumbai-headquartered Glenmark Pharmaceuticals will make a separate application to state regulators to get a manufacturing license under the provisions of the Drugs and Cosmetics Act and its rules.