Cough Syrup manufacturers will have to get samples tested and cleared by the government-certified laboratories before they can be exported overseas from June 1, announced the Directorate General of Foreign Trade on Monday. This decision comes after quality concerns were raised globally for India-made cough syrups that were linked to dozens of deaths in Gambia and Uzbekistan last year.
The notification by the DGFT said, "Cough Syrup shall be permitted to be exported subject to the export sample being tested and production of the Certificate of Analysis issued by any of the laboratories," as mentioned in the notification.
What labs are approved by the Centre?
The specified central government labs include National Accreditation Board for Testing and Calibration Laboratories, Indian Pharmacopoeia Commission, central drugs lab in Kolkata, regional testing lab in Chandigarh and Guwahati and central drug testing lab in Chennai, Mumbai and Hyderabad.
The Ministry of Health and Family Welfare would work with the exporters in order to ensure smooth implementation of this new notification.
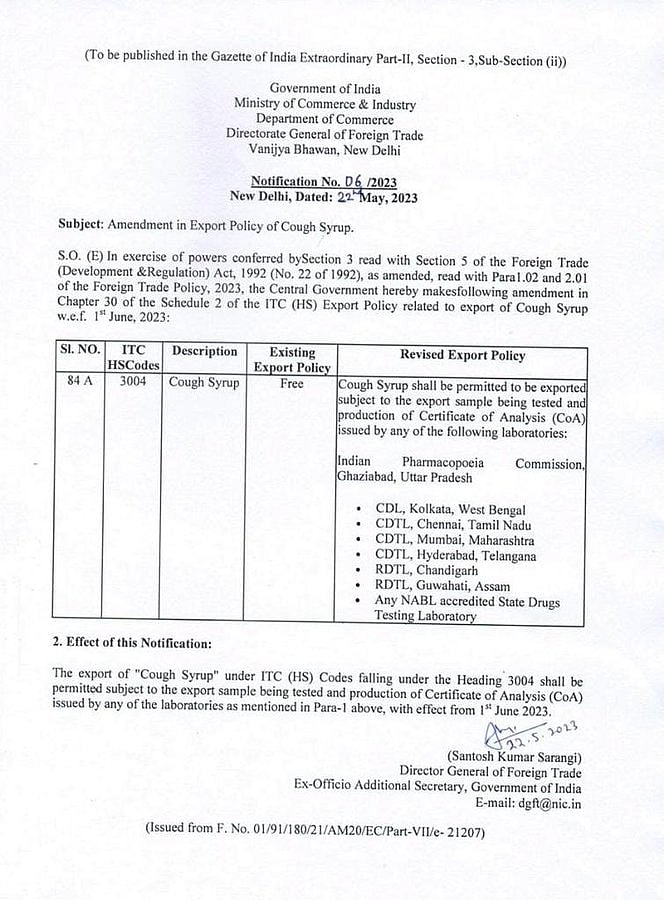
Notification from DGFT |
Pharmaceutical industry under duress due to deaths abroad
India's pharmaceutical industry that was valued at $41 billion experienced a major setback after Indian cough syrup was connected with the death of close to 70 children in Gambia and 18 in Uzbekistan.
In the last fiscal the exports of cough syrup was at $17.6 billion which was higher than the exports in the 2021-22.





